General Program Questions
Data Collection
Data Use and Privacy
Educational Webinars
Eligibility
Implementation
GENERAL PROGRAM QUESTIONS
Back To Top
1. What are the benefits of participating?
The Agency for Healthcare Research and Quality (AHRQ) Safety Program for MRSA Prevention National Program Team (NPT) will work closely with your long-term care facility staff to develop or enhance the approaches your facility takes to optimize skin care and infection prevention. Using evidence-based, scientific, practical implementation strategies, we can help members of your long-term care facility promote best practices and compliance with bathing practices, hand hygiene, prevention of methicillin-resistant Staphylococcus aureus (MRSA) and other Multidrug Resistant Organisms (MDROs), environmental cleaning and more.
By participating, your facility will—
- Improve resident skincare and bathing practices
- Promote and optimize care of resident wounds and pressure injuries
- Focus on MRSA and other MDRO transmission prevention
- Enhance teamwork and communication
- Improve resident safety and safety culture
Our team of subject matter experts has expertise in both MRSA prevention and team building and will be readily accessible for coaching, technical assistance, and ongoing education. Visit the Eligibility FAQ section or sign up for an Informational Webinar to find out if you qualify to participate in the program.
2. How does this program enhance teamwork and communication?
This program promotes communication, teamwork, and leadership engagement to support a culture of resident safety. It combines clinical best practices with systems improvements to resident safety culture.
The program is implemented by a team focused on quality improvement and patient safety, committed to meeting monthly to discuss the project and the interventions, and other patient safety concerns.
3. Who is sponsoring the program?
This program is funded and guided by AHRQ, which is part of the U.S. Department of Health and Human Services. Johns Hopkins Medicine’s Armstrong Institute for Patient Safety and Quality is conducting the work in collaboration with NORC at the University of Chicago.
4. What does it cost our facility to participate in the program?
There is no cost to participate in the program. Participating facilities will not incur any fees to receive assistance as a part of this program and will not receive any payment for participation.
5. Are continuing education credits available for participation in this program?
Continuing education credits are available as follows:
CME, CEU, and NAB credits will be available for participating physicians, nurses and nurse practitioners, physician assistants, and long-term care facility administrators. Infection Preventionists can use CME credits earned through this program as equivalent credits toward their CBIC recertification.
6. What is the commitment for the program?
This program asks for an 18-month commitment. Orientation webinars to introduce the program and train representatives from your long-term care facility about MRSA prevention will begin in June 2023. The following table shows long-term care facility tasks and due dates.
Task
|
Submission Date
|
|
Review and sign the Letter of Commitment for facility participation in the Safety Program.
|
July 2023
|
|
Assemble a multidisciplinary core team for your long-term care facility. The team could consist of a clinician champion, nurse champion, or infection preventionist to lead and coordinate local efforts while participating in the AHRQ Safety Program and sustain those efforts after the program is completed. Each facility also will identify a core team lead and/or data coordinator who will facilitate data collection at your site. The National Program Team will be available to assist facilities in forming their core team.
|
June 2023 –
July 2023
|
|
Ensure that members of your facility have access to the Safety Program website. This website hosts all educational content associated with the Safety Program.
|
June 2023 –
July 2023
|
|
Submit monthly infection prevention data for the 12 months prior to the start of the program (June 2022–May 2023). A facility lead or data coordinator can submit data.
|
October 2023
|
|
Participate in educational programs, including an orientation webinar and monthly educational webinars. Team leaders are strongly encouraged to participate in the live webinars. Other staff members are encouraged to join live webinars, or at a minimum, access and engage with content (e.g., recorded presentations, educational materials for staff, clinicians, and residents) on the program website.
|
June 2023 – November 2024
|
|
Meet regularly as a team (e.g., monthly) to discuss the content of the webinars, review current infection prevention practices, and identify areas for improvement in your facility.
|
June 2023 – November 2024
|
|
Submit monthly infection prevention data. A team lead or data coordinator can submit data.
|
June 2023 – November 2024
|
7. What is required of our facility to participate in the program?
Each facility will identify a core team, including a team lead (e.g., clinician or nursing champion) and an infection preventionist to assist with overseeing work. Each facility will also identify one team member who will facilitate data collection at your site. Activities include the following—
- Participate in regular webinars (30–60 minutes each, once or twice a month).
- Determine and implement approaches to promote and improve skin care, pressure injury prevention and infection prevention. Using approaches discussed in the webinars, facility teams will develop and implement strategies including bathing practices, wound and pressure injury care, hand hygiene, enhanced barrier precautions, environmental cleaning, and antibiotic stewardship.
- In addition, teams will practice using process measure auditing tools, checklists, guides for performing mini-root–cause analyses for healthcare-associated infections (HAIs), tools to assess the thoroughness of environmental cleaning, and implementation guides for MRSA surveillance and decolonization.
- Collect and review data. Your team is encouraged to work together to review and submit monthly data extracts of infection and transfer rates for facility resident(s) to an acute care hospital with reason of suspected or confirmed infection, as well as the program provided benchmark reports that summarize deidentified data from similar groups.
8. How much time does this program take?
We anticipate that participating long-term care facility leads will likely need to spend a minimum of 3 hours per month on the AHRQ Safety Program for MRSA Prevention. Approximately 1 hour would be devoted to participating in webinars. The remaining hours would be spent making the changes discussed in the webinars or calling into office hours. Time will be necessary to collect, submit, and review data.
9. When is the deadline to enroll?
The deadline to enroll in the long-term care cohort is July 31, 2023. We encourage you to submit an application to join the program as soon as possible as space in the program will be limited.
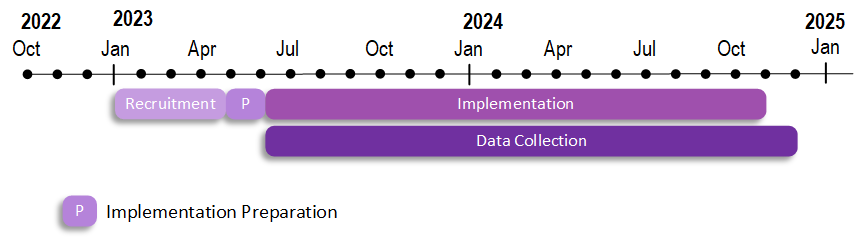
10. What is the timeline for the program?
Preparation for the long-term care cohort will begin in May 2023, and program implementation will begin in June 2023 (June 2023 through November 2024).
11. Is a contract required to participate in the program?
No, a contract is not required to participate in the program. To apply for the program, your facility lead or other primary contact will need to complete the online application form available on the Join the Program page.
The program requests a Letter of Commitment signed by a facility administrator, the director of nursing, and the medical director. This is not a contract; rather, it is an agreement to work with us. We ask for these signatures to ensure support for the program from the facility’s leadership and to approve the minimum time commitment of 3 hours per month. However, the actual program commitments/requirements will be fulfilled by the facility’s participating staff.
12. We already employ the resident care strategies above. How can joining this program improve our MRSA infection rates and contribute to greater sustained adoption?
In addition to using evidence-based, scientific, practical implementation strategies, the program employs a customizable quality improvement method that promotes communication, teamwork, and leadership engagement to support a culture of resident safety.
Long-term care teams will also practice using auditing tools, checklists, guides for performing mini-root cause analyses for healthcare-associated infections (HAIs), and other system-based changes that can prevent skin and soft issue infections. The program combines clinical best practices and enhanced teamwork and communications with systems improvements to the culture of resident safety.
13. Is a data use agreement (DUA) required to participate in the program?
No, a DUA is not required for participation in the program. Since the program only requests deidentified information and does not request any protected health information, program participation does not require a data use agreement. However, if your facility determines that it still needs a DUA to define the transaction, we can provide a template for your review.
14. What materials do I need to complete before the program begins?
You need to complete the online application and a Letter of Commitment. The application requests general facility demographic information, as well as contact information. The Letter of Commitment should be signed by a facility administrator, the director of nursing, and the medical director.
Johns Hopkins Medical Institutional Review Board (IRB) has determined this program is not human subjects research (Johns Hopkins IRB #IRB00284760). This program does not involve human subjects research, so no institutional review board (IRB) approval is required. Your facility should be able to frame this program as a quality improvement study. If your facility requires an individual IRB submission for informational purposes, we will be glad to help you as needed.
DATA COLLECTION
Back To Top
15. What data are being collected?
Please see the tables below.
Data Collection Tools
Tool
|
Purpose
|
To Be Completed by
|
Frequency of Data Collection
|
|
Nursing Home Survey on Patient Safety Culture (NHSOPS)
|
To collect information on resident safety issues
|
Facility staff
|
Once at the beginning of the program
Once post-implementation (month 18)
|
|
Infrastructure Assessment (Gap Analysis)
|
To evaluate existing resources and processes and identify areas of improvement to facilitate interventions to reduce infections caused by methicillin-resistant Staphylococcus aureus (MRSA)
|
Facility Lead or Infection Preventionist
|
Once at the beginning of the program
Once post-implementation (month 18)
|
|
Implementation Assessment (Team Checkup Tool)
|
Checklist of key actions to guide staff members towards a culture of safety by using guidelines, tools, and resources
|
One staff member per long-term care facility
|
Monthly
|
To evaluate the changes in infection prevention during the 18-month program, your facility's core team lead or data coordinator will be asked to submit the clinical outcomes data outlined in the tables below.
We estimate the initial data pull will take 2-3 hours, and subsequent monthly data pulls will take 30 minutes each. Facilities that do not have electronic health record technical support or strong internet access to upload files will be offered a fax option to provide their data.
The program team will offer flexibility around data collection dates to minimize barriers to program participation. The program team will work with your facility to determine a data collection schedule that will work best for you.
Electronic Health Record Clinical Data Extracts